Automatic Localization and Classification of Coronary Artery Plaques from Cardiac CTA with A Boundary-Constrained 3D Fully Convolutional Network
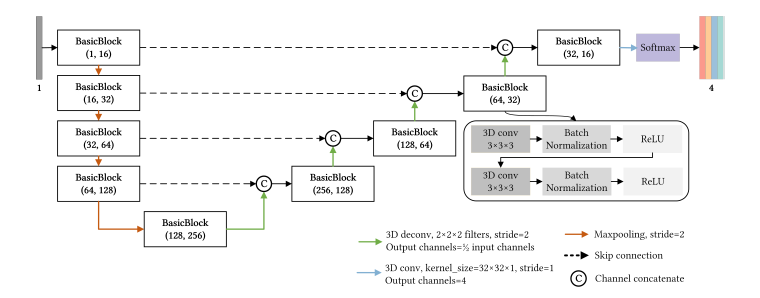
Abstract—Atherosclerotic plaques are the deposits of cholesterol and other substances in the walls of the arteries, whose location and categories are significant for risk assessment, diagnosis, and treatment of coronary artery diseases (CAD). Coronary computed tomography angiography (CCTA) is an effective non-invasive tool for plaque localization and classification. Manual assessment for plaques is time-consuming, and results vary due to differences in the observers. Thus, automatic methods with machine learning or deep learning technologies have been explored. However, existing learning-based methods have limited performance in recognizing non-calcified plaques, locating plaque areas accurately, and producing continuous predictions for long-range plaques due to the severe class imbalance, similarity between foreground and background, and lesion appearance variability. To overcome the difficulties mentioned above, we propose a method for automatic plaque localization and classification, including three types, namely, non-calcified plaques (NCAP), mixed calcified plaques (MCAP), and calcified plaques (CAP), which based on a boundary constrained 3D fully convolutional network. First, the curved planar reformation (CPR) technique is utilized to reformat coronary artery voxels into straightened volumes, guided by coronary artery centerlines extracted by Mimics from CCTA, to obtain clearer perspective of plaques and exclude most of the background area. Then, the fully convolutional network that leverages encoding-decoding architecture with skip connections is trained to detect plaque subtypes in vessel segments, which enables entire artery predictions with less calculating redundancy. To improve the accuracy and continuity of detected plaque regions, the signed distance map (SDM) is utilized to construct the loss function by means of adding boundary constraints and emphasizing the miscalculation among different lesion types. Our method is evaluated on a dataset of 33 patients’ CCTA scans and achieves the best macro f1 score of 0.798 and MCC of 0.747, an improvement of at least 5% in f1 score for each plaque type compared with other methods. In addition, the runtime for proposed method is only 0.081s per branch, one fifth of the comparison method. We further investigated the boundary constraints through an ablation experiment, which demonstrates the method’s effectiveness in improving the boundary accuracy, plaque range continuity and mitigating the class imbalance problem.